The effect of transcutaneous median nerve stimulation on heart rate variability in healthy young adults
Introduction
While heart rate (HR) regulation by the autonomic nervous system is maintained within a narrow range, beat-to-beat time analysis demonstrates high variability among healthy adults (1). This can be detected through analysis of HR variability (HRV) (2)—a term that refers to the variation in time between consecutive heartbeats and that particularly reflects the effect of the parasympathetic system on the heart (3).
HRV has been shown to be associated with cardiovascular diseases (CVDs) (4). An unbalanced autonomic tone (i.e., increased sympathetic and decreased parasympathetic tones) was linked to increased risk of morbidity and mortality from CVD (5-7). In a largescale longitudinal study (N=11,715; conducted over 19.4 years), researchers demonstrated that cardiac autonomic dysfunction, denoted by low resting short-term HRV, was associated with a higher atrial fibrillation incidence (8). An additional largescale study (N=1,141) showed that low HRV parameters were significantly and independently associated with congestive heart failure incidences (9). In addition, low HRV was associated with old age (10), increased HR, beta-blocker use, diuretic use, and cigarette smoking (11).
The relationships found between low HRV and CVD raises the possibility of enhancing HRV in order to lower the risk of CVD. Increased vagal tone induced by exercise was shown to increase HRV, and was speculated to improve cardiovascular health (12). A variety of additional means for enhancing HRV were studied in different populations. Practicing yoga (13) and Tai Chi (14) were shown to increase HRV in healthy participants. High-intensity interval training increased HRV in chronic heart failure patients (15). Symmetric breathing with an equal inhaling/exhalation ratio, or an uneven breathing pattern with longer exhalation than inhalation, both increased HRV in healthy people (16). Moreover, the effects of the outdoor environment on HRV were evaluated in healthy participants (17,18). In addition, a review on studies evaluating Nei Guan acupuncture showed its effect on HRV in healthy and non-healthy participants (19).
An additional method for increasing HRV is invasive vagal nerve stimulation (VNS), which was approved in Europe and in the USA as a chronic therapy for depression (20) and epilepsy (21,22). In heart failure patients, 6 months of VNS therapy (implanted on the left or right side of the vagus nerves) increased HRV (23).
Non-invasive techniques of VNS were also applied, mostly by an electrode placed on the ear or the neck (24). Ear transcutaneous VNS (tVNS) treatment for 20 minutes (200 ms, 30 Hz) in healthy humans increased HRV compared to sham treatment (25). Moreover, tVNS of the left cymba conchae reduced HR, affected cardiac and peripheral autonomic control, and increased the responses of peripheral autonomic control to orthostatic stress (26). In their recent study (24), conducted an extensive review of the effects of non-invasive vagus nerve stimulation of the ear skin in humans on HRV. The researchers concluded that the findings produced mixed results. Finally, transcutaneous electrical nerve stimulation (TENS) in the paravertebral ganglionar region (from T1 to L2) modulates sympathetic and parasympathetic activity in a frequency-dependent manner in young healthy participants (27).
An additional location used to stimulate the vagus in animal models is in the median nerve. The effects of median nerve stimulation (MNS) on cardiac activity were evaluated in animal models. In cats, the median nerves in both forelimbs were exposed, and were directly stimulated by stainless steel bipolar electrodes placed around each nerve (28). In rabbits, direct MNS affected the left stellate ganglion, resulting in the prevention of ventricular arrhythmias; nevertheless, there were no effects on HR or blood pressure (BP) (29). In dogs, direct stimulation of the median nerve in the left forelimb was shown to enhance vagal nerve activity. It was speculated that MNS increased spinal cord activity, then decreased the cardiac sympathetic activity, and finally increased the cervical vagus nerve activity (30).
Sun et al. [2015] tested the effect of transcutaneous electrical acupuncture stimulation at PC6, for 30 minutes over a four-day period in healthy male subjects before and after head-down bed rest. The researchers found that in the treated group, low frequency (LF) and LF/high frequency (HF) ratio were increased after bed rest compared with before, and concluded that stimulation at PC6 may be a novel therapeutic method for the prevention or treatment of autonomic dysfunction (31).
Moreover, stimulation of the P6 point, in healthy young men working night shifts, improved HF and LF parameters of HRV (32), yet the stimulation entailed laser light energy rather than electric stimulation. In a recent study, researchers evaluated different frequencies of MNS on HRV in healthy men, showing that 120 Hz increased root-mean square differences of successive RR intervals (RMSSD) (33).
Considering the positive effects of MNS on autonomic balance as indicated by HRV parameters and its relations to improved health, it is desirable to find methods to increase HRV, mainly in people who have low values. Thus, the aim of the study was to investigate a new method for increasing HRV via transcutaneous MNS in the left hand in subjects with low HRV [standard deviation of NN interval (SDNN) lower than 40].
Methods
Participants
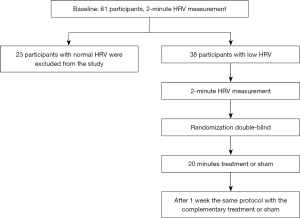
Sixty-one healthy adults were invited to take part in the study, contacted via an alternative sports and health center. The participants received in-depth explanations about the research and then signed an informed written consent form. After measuring HRV for all 61 adults, 38 participants (18 men, 20 women) with lower than 40 ms SDNN were included in the study (mean age 49.5±6.8 years).
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hillel Yafe Medical Center’s Helsinki Committee (# 0024-18-HYMC). Informed consent was taken from all individual participants.
Procedure
The study was a randomized, double-blind, crossover research study. Participants with low HRV were treated with real or sham MNS, as seen in Figure 1. As a baseline, the 61 participants were measured for HRV variables, BP, and HR, and were asked about chronic diseases, medication, and physical activity.
The participants were asked to visit the lab twice, with at least seven days between the two sessions. They were asked to refrain from eating, drinking coffee, and physical activity for the 2 hours prior to each session. At the start of their first visit to the lab, the participants were given an in-depth explanation of the procedure and were asked to sign an informed written consent form. Next, their systolic and diastolic BPs (SBP and DBP) were measured after a 10-minute rest while sitting on a chair, followed by a 2-minute recording of their HR for the HRV measurement. Participants with SDNN lower than 40 ms were excluded from the study at this point. Included participants were fitted with a polar belt on their chest, and a TENS device on their left wrist [ReguRate (RR2) neurostimulation system], and after 10 more minutes of rest, another 2-minute HRV measurement was performed. Next, the participants were randomly administered either active or sham treatment procedure, in which the participant and the researcher who measured the physiological parameters were blind to the treatment. After at least one week the participants were asked to come again and complete HRV measures before and after treatment which was opposed to the treatment in the first session.
Intervention
Active treatment
A TENS (ReguRate RR2 neurostimulation system) was placed on the left palmar surface of the wrist and activated for 20 minutes (5 Hz, 100–300 µs). Stimulation intensity (mA) was adjusted individually for each subject to the sub-motor response threshold.
Sham treatment
A TENS device was placed on the left dorsal surface of the wrist for 20 minutes but was not activated. If participants had received active treatment in the first session and were then administered sham treatment in the second session, they were told that different locations of the device cause different sensations, ranging from no sensation at all, to a tingling sensation in the fingers—to prevent their understanding that this was a sham treatment.
Measurements
Measurements were conducted at three time points: immediately before receiving [pre-treatment (PT)]; immediately after being administered treatment (post-treatment: PT0); and 10 minutes post-treatment (PT1). The measurements included: (I) electrocardiogram (ECG), recorded using wireless “Wecardio ECG Event Record” device (Shenzhen, China). Recorded data were analyzed by the Wecardio Borsam Company (Shenzhen, China); (II) HR, measured by the Polar (Kempele, Finland) watch H10; (III) BP, measured by the OMRON BP monitor (Kyoto, Japan); and (IV) the Visual Analogue Scale (VAS) Questionnaire (34), which was completed by the participants after the first treatment. The participants were asked to rate the degree to which they had experienced each of the following 11 sensations during the session—numbness, itching, burning, contraction, nausea, headache, dizziness, shortness of breath, chest pain, difficulty concentrating, and fatigue—on a scale of 1 (no sensation) to 10 (severe sensation).
Statistical analysis
Sample size was calculated to be 60 using G*Power version 3.1.9.2 program with a power of 80% to detect a 20% difference between means of SDNN component and α=0.05. A description of the participants’ means and standard deviations for normally distributed variables, and the median and interquartile range (IQR) for non-normally distributed variables, are presented. The normally distributed variables were analyzed using repeated measures analysis of variance (ANOVA) time (PT, PT0, and PT1) X situation (treatment, control). LF and HF had non-normal distribution and were analyzed using nonparametric tests (Mann-Whitney & Friedman tests). The characteristics of the participants with higher than 40 SDNN ms and who had been excluded from the treatment sessions (n=23) were compared to those of the treatment group (n=38), including age, gender, body mass index (BMI), medication, resting HR, BP, physical activity habits, and HRV. Statistical analyses were performed using SPSS, v.25 (SPSS Inc., Chicago, IL, USA). A P value of ≤0.05 was considered statistically significant.
Results
The differences in characteristics between the excluded participants and the included ones are presented in Table 1. The excluded participants were significantly younger, had lower SBP, DBP, and HR during rest, and as anticipated, all HRV measures were higher.
Table 1
| Variables | Included | Not included |
|---|---|---|
| Male/female | 18/20 | 8/15 |
| Age (years)* | 49.4±6.8 | 36.5±9.2 |
| BMI, kg/m2 | 25.4±3.7 | 23.9±4.3 |
| Medication | ||
| Yes | 6 (15.8) | 3 (13.0) |
| No | 32 (84.2) | 20 (87.0) |
| Physical activity | ||
| Inactive | 12 (31.6) | 7 (30.4) |
| Light | 19 (50.0) | 8 (34.8) |
| Moderate | 6 (15.8) | 7 (30.4) |
| Very active | 1 (2.6) | 1 (4.3) |
| Activity type | ||
| Aerobic only | 12 (31.6) | 1 (4.3) |
| Yoga/pilates only | 2 (5.3) | 2 (8.7) |
| Strength only | 3 (7.9) | 7 (30.4) |
| Combined (aerobic/strength/yoga, pilates) | 10 (26.3) | 6 (26.1) |
| SBP (mmHg)* | 127.2±16.0 | 116.1±9.1 |
| DBP (mmHg)* | 81.9±10.8 | 71.3±7.6 |
| HRrest (bpm)* | 74.5±10.8 | 66.3±6.6 |
| SDNN (ms)* | 25.8±7.6 | 58.0±16.9 |
| RMSSD (ms)* | 20.7±8.6 | 51.6±19.5 |
Data are presented as number, mean ± standard deviation, or n (%). *, P<0.05. SDNN, standard deviation of NN intervals; BMI, body mass index; SBP, systolic blood pressure; DBP, diastolic blood pressure; HRrest, heart rate at rest; RMSSD, root mean square of successive RR interval differences.
The values of the HRV parameters, BP and resting HR are presented in Tables 2-4. No significant difference was found between the active and sham treatment for HRV variables or BP and HR values. Moreover, no interaction was found for any of the variables (P>0.05). Statistically significant yet small differences were found for the time factor: SBP (P=0.036), SDNN (P=0.025) and HR (P=0.001). However, these were clinically non-significant differences.
Table 2
| Variables | Active treatment | Sham treatment | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post 1 | Post 2 | Pre | Post 1 | Post 2 | ||
| SDNN (ms) | 26.7 (8.4) | 28.5 (10.2) | 28.0 (11.1) | 25.4 (8.1) | 29.0 (11.1) | 29.5 (10.4) | |
| RMSSD (ms) | 21.4 (10.1) | 23.3 (11.3) | 20.9 (10.2) | 20.6 (10.3) | 20.8 (8.4) | 20.6 (8.0) | |
| SBP (mmHg) | 126.8 (16.4) | 125.8 (17.0) | 123.5 (17.3) | 123.6 (15.3) | 121.7 (18.1) | 121.5 (16.6) | |
| DBP (mmHg) | 80.2 (10.7) | 79.5 (10.8) | 80.6 (10.1) | 79.5 (10.9) | 78.6 (13.2) | 79.6 (10.6) | |
| HRrest (bpm) | 74.9 (10.4) | 72.2 (9.5) | 72.7 (9.0) | 74.8 (10.7) | 73.6 (9.2) | 73.5 (8.8) | |
Data are presented as mean (SD). Post 1 indicates values measured immediately after treatment; Post 2 indicates values measured 10 minutes after treatment. HRV, heart rate variability; HR, heart rate; SDNN, standard deviation of NN intervals; RMSSD, root mean square of successive RR interval differences; SBP, systolic blood pressure; DBP, diastolic blood pressure; HRrest, heart rate at rest; SD, standard deviation.
Table 3
| Variables | Active treatment | Sham treatment | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post 1 | Post 2 | Pre | Post 1 | Post 2 | ||
| LF (ms2) | 169.4 (146.7) | 282.1 (334.9)* | 226.3 (193.6)* | 137.9 (109.2) | 204.6 (155.2)* | 307.0 (725.4) | |
| HF (ms2) | 152.3 (167.5) | 166.2 (170.7) | 136.4 (147.4) | 170.1 (301.2) | 124.3 (108.6) | 247.7 (809.2) | |
Data are presented as mean (SD). *, P<0.05. Post 1 indicates values measured immediately after treatment; Post 2 indicates values measured 10 minutes after treatment. HRV, heart rate variability; LF, low frequency; HF, high frequency; SD, standard deviation.
Table 4
| Variables | Active treatment | Sham treatment | |||||
|---|---|---|---|---|---|---|---|
| Pre | Post 1 | Post 2 | Pre | Post 1 | Post 2 | ||
| LF (ms2) | 98 [130] | 192 [297.5] | 139 [209.5] | 117 [151] | 116 [283] | 148 [240.5] | |
| HF (ms2) | 87 [185.5] | 121 [169.5] | 95 [160] | 112 [158] | 110 [132] | 88 [94.5] | |
Data are presented as median [IQR]. Post 1 indicates values measured immediately after treatment; Post 2 indicates values measured 10 minutes after treatment. LF: P=0.96; HF: P=0.29. HRV, heart rate variability; LF, low frequency; HF, high frequency; IQR, interquartile range.
Based on the questionnaire completed after the first session, participants who were administered active treatment (n=21) reported the following: six participants reported fatigue (28.6%); three participants reported having difficulty concentrating (14.3%); two reported having a headache; one reported itching, one reporting a feeling of numbness; and one reported nausea. No other sensations during treatment were reported. Following the sham treatment (n=17), two participants reported experiencing numbness (11.7%) and another reported experiencing contractions.
Discussion
Low HRV SDNN parameter in healthy adults was measured in 62% of the participants. The attempt to increase the participants low HRV by the new method of transcutaneous MNS did not show effect as compared to sham treatment. These results are not in accordance to a study on heart transplant patients who were administered a single 40 minutes session of trans-cutaneous electrical stimulation, at an intensity of 0.8–1.9 mA and a frequency of 5–30 Hz, at acupoints PC5 and PC6 (as P6 and P5 overlie the deep median nerve). The treatment was found to acutely improve HRV measures in the transplant patients and enhance the sympathovagal index during its application. It was therefore concluded that electrical stimulation at PC5 and PC6 acutely modulates HRV in transplant patients (35). The ineffectiveness of the current methods might be related to parameters of the treatment or of the population. In an earlier study, the effect of short vagal stimulation, 10 minutes on the right ear versus the left ear was examined. The researchers found that only stimulation of right ear increased SDNN in healthy subjects. Long duration, one hour stimulation of the right ear increased LF and LF/HF components of HRV, and SDNN in women, but not in men (36). Our study included both male and female which may affect the results. Also, different parameters of MNS such as the frequency, showed to have different effects on HRV (33)
The effect of non-invasive electric stimulation of the median nerve on HRV parameter clearly needs more evaluation. The safety of the MNS treatment in our study was evaluated by monitoring side effects and by using a VAS questionnaire after the first treatment session. No serious adverse side effects were noted. In the active treatment, 28.6% of the participants reported feeling fatigue and 14.3% reported difficulty in concentration. No fatigue or difficulty in concentration during the sham treatment was reported. TENS is considered safe, with relatively minor side effects, even for prolonged use (37). A recent review on the safety of tVNS in humans indicated different side effects, such as local skin irritation, headache and nasopharyngitis, most of them were tolerable or infrequent (38).
Conclusions
The effect of transcutaneous electrical MNS, as applied in the present study, did not differ from the effect of sham treatment on HRV on healthy people with low HRV. It is possible that stronger or longer stimulation may have a greater effect on HRV parameters. Effective stimulation with MNS via a portable and convenient device could be a treatment that people administer themselves as part of their routine. Yet additional investigation is needed for evaluating the possible clinical applications or effectiveness of short term non-invasive electrical stimulation of the median nerve on healthy subjects.
Acknowledgments
Funding: None.
Footnote
Data Sharing Statement: Available at https://ht.amegroups.com/article/view/10.21037/ht-23-5/dss
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://ht.amegroups.com/article/view/10.21037/ht-23-5/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). The study was approved by the Hillel Yafe Medical Center’s Helsinki Committee (# 0024-18-HYMC). Informed consent was taken from all individual participants.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- McCraty R, Shaffer F. Heart Rate Variability: New Perspectives on Physiological Mechanisms, Assessment of Self-regulatory Capacity, and Health risk. Glob Adv Health Med 2015;4:46-61. [Crossref] [PubMed]
- Shaffer F, Ginsberg JP. An Overview of Heart Rate Variability Metrics and Norms. Front Public Health 2017;5:258. [Crossref] [PubMed]
- Kemp AH, Koenig J, Thayer JF. From psychological moments to mortality: A multidisciplinary synthesis on heart rate variability spanning the continuum of time. Neurosci Biobehav Rev 2017;83:547-67. [Crossref] [PubMed]
- Kubota Y, Chen LY, Whitsel EA, et al. Heart rate variability and lifetime risk of cardiovascular disease: the Atherosclerosis Risk in Communities Study. Ann Epidemiol 2017;27:619-625.e2. [Crossref] [PubMed]
- Ponikowski P, Anker SD, Chua TP, et al. Depressed heart rate variability as an independent predictor of death in chronic congestive heart failure secondary to ischemic or idiopathic dilated cardiomyopathy. Am J Cardiol 1997;79:1645-50. [Crossref] [PubMed]
- Ginty AT, Kraynak TE, Fisher JP, et al. Cardiovascular and autonomic reactivity to psychological stress: Neurophysiological substrates and links to cardiovascular disease. Auton Neurosci 2017;207:2-9. [Crossref] [PubMed]
- Chida Y, Steptoe A. Greater cardiovascular responses to laboratory mental stress are associated with poor subsequent cardiovascular risk status: a meta-analysis of prospective evidence. Hypertension 2010;55:1026-32. [Crossref] [PubMed]
- Agarwal SK, Norby FL, Whitsel EA, et al. Cardiac Autonomic Dysfunction and Incidence of Atrial Fibrillation: Results From 20 Years Follow-Up. J Am Coll Cardiol 2017;69:291-9. [Crossref] [PubMed]
- Patel VN, Pierce BR, Bodapati RK, et al. Association of Holter-Derived Heart Rate Variability Parameters With the Development of Congestive Heart Failure in the Cardiovascular Health Study. JACC Heart Fail 2017;5:423-31. [Crossref] [PubMed]
- Choi JB, Hong S, Nelesen R, et al. Age and ethnicity differences in short-term heart-rate variability. Psychosom Med 2006;68:421-6. [Crossref] [PubMed]
- Tsuji H, Venditti FJ Jr, Manders ES, et al. Determinants of heart rate variability. J Am Coll Cardiol 1996;28:1539-46. [Crossref] [PubMed]
- Routledge FS, Campbell TS, McFetridge-Durdle JA, et al. Improvements in heart rate variability with exercise therapy. Can J Cardiol 2010;26:303-12. [Crossref] [PubMed]
- Praveena SM, Asha G, Sunita M, et al. Yoga Offers Cardiovascular Protection in Early Postmenopausal Women. Int J Yoga 2018;11:37-43. [Crossref] [PubMed]
- Cole AR, Wijarnpreecha K, Chattipakorn SC, et al. Effects of Tai Chi exercise on heart rate variability. Complement Ther Clin Pract 2016;23:59-63. [Crossref] [PubMed]
- Besnier F, Labrunée M, Richard L, et al. Short-term effects of a 3-week interval training program on heart rate variability in chronic heart failure. A randomised controlled trial. Ann Phys Rehabil Med 2019;62:321-8. [Crossref] [PubMed]
- De Couck M, Caers R, Musch L, et al. How breathing can help you make better decisions: Two studies on the effects of breathing patterns on heart rate variability and decision-making in business cases. Int J Psychophysiol 2019;139:1-9. [Crossref] [PubMed]
- Horiuchi M, Endo J, Takayama N, et al. Impact of viewing vs. not viewing a real forest on physiological and psychological responses in the same setting. Int J Environ Res Public Health 2014;11:10883-901. [Crossref] [PubMed]
- Stigsdotter UK, Corazon SS, Sidenius U, et al. It is not all bad for the grey city - A crossover study on physiological and psychological restoration in a forest and an urban environment. Health Place 2017;46:145-54. [Crossref] [PubMed]
- Chung JW, Yan VC, Zhang H. Effect of acupuncture on heart rate variability: a systematic review. Evid Based Complement Alternat Med 2014;2014:819871. [Crossref] [PubMed]
- Albert U, Maina G, Aguglia A, et al. Vagus nerve stimulation for treatment-resistant mood disorders: a long-term naturalistic study. BMC Psychiatry 2015;15:64. [Crossref] [PubMed]
- Oliveira TVHF, Francisco AN. The role of vagus nerve stimulation in refractory epilepsy. Arq Neuropsiquiatr 2017;75:657-66. [Crossref] [PubMed]
- Clarke BM, Upton AR, Griffin H, et al. Chronic stimulation of the left vagus nerve in epilepsy: balance effects. Can J Neurol Sci 1997;24:230-4. [Crossref] [PubMed]
- Premchand RK, Sharma K, Mittal S, et al. Autonomic regulation therapy via left or right cervical vagus nerve stimulation in patients with chronic heart failure: results of the ANTHEM-HF trial. J Card Fail 2014;20:808-16. [Crossref] [PubMed]
- Burger AM, D'Agostini M, Verkuil B, et al. Moving beyond belief: A narrative review of potential biomarkers for transcutaneous vagus nerve stimulation. Psychophysiology 2020;57:e13571. [Crossref] [PubMed]
- Clancy JA, Mary DA, Witte KK, et al. Non-invasive vagus nerve stimulation in healthy humans reduces sympathetic nerve activity. Brain Stimul 2014;7:871-7. [Crossref] [PubMed]
- Tobaldini E, Toschi-Dias E, Appratto de Souza L, et al. Cardiac and Peripheral Autonomic Responses to Orthostatic Stress During Transcutaneous Vagus Nerve Stimulation in Healthy Subjects. J Clin Med 2019;8:496. [Crossref] [PubMed]
- Stein C, Dal Lago P, Ferreira JB, et al. Transcutaneous electrical nerve stimulation at different frequencies on heart rate variability in healthy subjects. Auton Neurosci 2011;165:205-8. [Crossref] [PubMed]
- Li P, Pitsillides KF, Rendig SV, et al. Reversal of reflex-induced myocardial ischemia by median nerve stimulation: a feline model of electroacupuncture. Circulation 1998;97:1186-94. [Crossref] [PubMed]
- Zhao S, Tang M, Yuan K, et al. Median nerve stimulation reduces ventricular arrhythmias induced by dorsomedial hypothalamic stimulation. J Interv Card Electrophysiol 2016;47:275-83. [Crossref] [PubMed]
- Zhao Q, Zhang S, Zhao H, et al. Median nerve stimulation prevents atrial electrical remodelling and inflammation in a canine model with rapid atrial pacing. Europace 2018;20:712-8. [Crossref] [PubMed]
- Sun J, Li X, Yang C, et al. Transcutaneous electrical acupuncture stimulation as a countermeasure against cardiovascular deconditioning during 4 days of head-down bed rest in humans. Acupunct Med 2015;33:381-7. [Crossref] [PubMed]
- Wu JH, Chen HY, Chang YJ, et al. Study of autonomic nervous activity of night shift workers treated with laser acupuncture. Photomed Laser Surg 2009;27:273-9. [Crossref] [PubMed]
- Maharjan A, Peng M, Cakmak YO. The effects of frequency-specific, non-invasive, median nerve stimulation on food-related attention and appetite. Appetite 2022;169:105807. [Crossref] [PubMed]
- Ablin JN, Odes L, Neumann L, et al. The Hebrew version of the FibroFatigue scale: validation of a questionnaire for assessment of fibromyalgia and chronic fatigue syndrome. Rheumatol Int 2010;30:1173-6. [Crossref] [PubMed]
- Moreira BR, Duque AP, Massolar CS, et al. Transcutaneous Electrical Stimulation of PC5 and PC6 Acupoints Modulates Autonomic Balance in Heart Transplant Patients: A Pilot Study. J Acupunct Meridian Stud 2019;12:84-9. [Crossref] [PubMed]
- De Couck M, Cserjesi R, Caers R, et al. Effects of short and prolonged transcutaneous vagus nerve stimulation on heart rate variability in healthy subjects. Auton Neurosci 2017;203:88-96. [Crossref] [PubMed]
- Johnson MI. Transcutaneous electrical nerve stimulation (TENS) as an adjunct for pain management in perioperative settings: a critical review. Expert Rev Neurother 2017;17:1013-27. [Crossref] [PubMed]
- Redgrave J, Day D, Leung H, et al. Safety and tolerability of Transcutaneous Vagus Nerve stimulation in humans; a systematic review. Brain Stimul 2018;11:1225-38. [Crossref] [PubMed]
Cite this article as: Tsuk S, Weiss G, Amedi R, Glixman O, Essel M, Grosman-Rimon L, Carasso R, Zeev A, Rotstein A. The effect of transcutaneous median nerve stimulation on heart rate variability in healthy young adults. Health Technol 2024;8:1.