
Maternal health risk analysis, automated pregnancy risk level identification and status monitoring system based on multivariable clinical data
Highlight box
Key findings
• The study found a strong correlation between high pregnancy risk and elevated systolic and diastolic blood pressures, higher blood sugar levels, and older ages.
• Among six machine learning models trained for pregnancy risk classification, the random forest classifier performed best, achieving a classification accuracy of 91%.
• A web-based system was developed to allow physicians to monitor pregnancy risk levels and clinical parameter trends.
What is known and what is new?
• Monitoring pregnancy health is crucial for early detection of complications, but low-resource settings face challenges such as the lack of specialist obstetricians and regular follow-up opportunities.
• This study introduces a machine learning-based system that can accurately classify pregnancy risk levels and monitor maternal health using a web-based interface, providing an efficient solution for managing complications in low-resource areas.
What is the implication, and what should change now?
• The machine learning-based system offers a reliable and accessible tool for healthcare providers in resource-limited settings to monitor pregnant women, improving early detection and management of potential complications.
• Further scaling and implementation of this system in low-resource healthcare settings is necessary to improve maternal care.
Introduction
Approximately 800 women die every day around the world due to pregnancy and child birth complications that can be prevented (1). Among these, 99% of the estimated global maternal mortality occurs in low resources setting, and half of this is in Sib-Saharan Africa (2-4). This is mainly due to many factors including inadequate healthcare setup, shortage of skilled health workers, poverty, distance to facilities, lack of information, poor quality service, cultural beliefs and practices, and various patient-related factors like the acceptability and affordability of maternal health services (1,5,6).
Primary complications during pregnancy and childbirth leading to maternal mortality and morbidity encompass severe bleeding, infections, high blood pressure, delivery-related complications, unsafe abortion, cardiovascular issues, diabetes, anemia, as well as depression and anxiety (7). The causes of maternal mortality in Sub-Saharan countries are post-partum hemorrhage, hypersensitive pregnancy disorder (e.g., pre-eclampsia/eclampsia), non-obstetric complications, and pregnancy-related infections (e.g., puerperal sepsis) (6). Pregnant women are also at a high risk of infectious disease such as coronavirus disease 2019 (COVID-19) and related complications (8,9). The disparity in maternal mortality reduction between developing and developed countries may indicate the low efficiency of evidence-based interventions in developing countries to detect and manage risk factors of maternal complications.
Several factors can contribute to high-risk pregnancy that leads to perinatal morbidity and mortality. Pregnancy risks can be high or low. High-risk pregnancies can cause a one in four chance of developing complications, while low-risk pregnancies have nearly a one in ten chance of developing complications (10). The mothers’ age, high blood pressure, obesity, diabetes, epilepsy, thyroid disease, and heart diseases are among the high-risk pregnancy complications (11-13). Early detection of pregnancy complications through regular quality examinations especially for high-risk pregnancies before, during and after childbirth can reduce maternal morbidity and mortality (14,15).
Risk assessment before and during pregnancy allows women and care givers to early predict the most likely adverse health events and make informed decisions to prevent it. It constitutes a crucial element of antenatal care (ANC) and is the central focus of maternal and childcare programs, holding the potential to enhance both maternal and perinatal outcomes (16-19). Various risk factor-based scoring or assessment systems are employed throughout the antepartum, intrapartum, and neonatal periods to stratify risks. Antenatal risk scores are numerical indices that reflect the collective risk associated with maternal characteristics. The first antenatal risk score was developed by Goodwin et al. (20) in 1969 and validated later by Burstyn (21) which was used to evaluate more than 900 pregnancies. The scores were classified into low, moderate, and high-risk categories for pregnancies. These risk scores play a crucial role in identifying pregnant women at high risk, thereby helping prevent maternal and fetal complications.
Obstetricians/physicians assess and categorize clinical data from clinical and diagnostic investigations to produce risk scores based on their knowledge and expertise. However, the existing approach is very subjective, necessitates interpretation abilities, and is prone to error which may lead to misdiagnosis. Furthermore, the manual assessment method could be tedious and time-consuming. Clinical decision support systems based on machine learning have evolved as a critical component of technology-assisted medical care, assisting numerous clinical procedures through the utilization of experiential knowledge. Clinical decision support system (CDSS) aims to enhance healthcare service by improving the decision-making process of healthcare professionals by supplementing medical choices with targeted clinical knowledge, patient data, and other health data (22,23).
The current work presents a development and validation of machine learning CDSS for pregnancy risk level assessment, pregnancy risk status monitoring system with the aim of allowing for improved pregnancy management and, as a result, better outcomes for both the mother and the fetus. We present this article in accordance with the TRIPOD reporting checklist (available at https://ht.amegroups.com/article/view/10.21037/ht-23-10/rc).
Methods
The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research did not involve humans, animals, or other subjects. According to Jimma University’s Institutional Review Board (IRB), no formal ethics approval was required in this particular case. Because of the retrospective nature of the research, the requirement for informed consent was waived.
The general framework of the proposed system is demonstrated in Figure 1. The proposed system includes a pregnancy risk level classification and status monitoring. The pregnancy risk predictor uses patient and clinical data to identify the risk level. The pregnancy risk status monitoring also uses the same attribute information and shows the trend line of each parameter from the start to current pregnancy period. Both of these systems are integrated and deployed into a web-based user interface.
Data
To deploy the proposed pregnancy risk predictor and monitoring system, a dataset comprising 1,014 observations was utilized. Each observation contained distinct attribute information, including age, systolic blood pressure, diastolic blood pressure, blood glucose level, body temperature, and heart rate. The data was gathered from a publicly available database (24) that was collected from various health facilities. Table 1 displays sample data from 10 pregnant women with annotated risk levels. The data consists of age (years at the time of pregnancy), systolic BP (upper blood pressure measurement in mmHg), diastolic BP (lower blood pressure measurement in mmHg), BS (blood glucose concentration in mmol/L), body temp (body temperature in Celsius), heart rate (resting heart rate in beats per minute), and risk level (categorized risk intensity during pregnancy).
Table 1
| No. | Age (years) | Systolic BP (mmHg) | Diastolic BP (mmHg) | BS (mmol/L) | Body temp (℃) | Heart rate (bpm) | Risk level |
|---|---|---|---|---|---|---|---|
| 1 | 25 | 130 | 80 | 15 | 36.6 | 86 | High risk |
| 2 | 35 | 140 | 90 | 13 | 36.6 | 70 | High risk |
| 3 | 35 | 120 | 60 | 6.1 | 36.6 | 76 | Low risk |
| 4 | 23 | 130 | 70 | 7.01 | 36.6 | 78 | Mid risk |
| 5 | 35 | 85 | 60 | 11 | 38.8 | 86 | High risk |
| 6 | 32 | 120 | 90 | 6.9 | 36.6 | 70 | Mid risk |
| 7 | 42 | 130 | 80 | 18 | 36.6 | 70 | High risk |
| 8 | 23 | 90 | 60 | 7.01 | 36.6 | 76 | Low risk |
| 9 | 19 | 120 | 80 | 7 | 36.6 | 70 | Mid risk |
| 10 | 25 | 110 | 89 | 7.01 | 36.6 | 77 | Low risk |
BP, blood pressure; BS, blood glucose.
Pregnancy risk factor analysis
Various risk factors play a role in pregnancy complications. This study examines major risk factors, including age, blood pressure (both systolic and diastolic), blood sugar level, body temperature, and heart rate. The analysis focuses on their contributions to high, medium, and low pregnancy risk levels. Box and whisker plots, a standardized method for visualizing data distribution using the five-number summary (minimum, first quartile, median, third quartile, and maximum), were used to represent the distribution of each risk level in relation to the collected data for each risk factor. In addition, to analyze the correlation of each risk factor with each other and with the high-risk level, a correlation matrix was used. Correlation matrix is a table that shows the correlation coeffects between variables.
Training and testing risk level prediction models
In this study, five machine learning models were trained and tested including K-nearest neighbor (KNN), multiclass logistic regression (MLR), support vector machine (SVM), extreme gradient boosting (XGBoost), and random forest—alongside a deep learning model, the multi-layer perceptron neural network (MLP), for classifying pregnancy risk levels. To evaluate the performance of each model and identify the best one, the same dataset was used for both training and testing across all classifiers.
KNN is a non-parametric, supervised algorithm that can be applied to both classification and regression tasks. For classification, it assigns a class to an object based on the majority vote of its nearest neighbors. Multiclass or multinomial regression extends logistic regression to handle problems with multiple classes by altering the loss function to cross-entropy and predicting a probability distribution across several outcomes. XGBoost, on the other hand, is an ensemble learning algorithm that uses decision trees within a gradient boosting framework. Known for its efficiency, flexibility, and scalability, it is a highly optimized distributed gradient boosting library. XGBoost applies machine learning algorithms within the Gradient Boosting framework, offering parallel tree boosting to improve efficiency, flexibility, and portability (25). For this paper, the XGBoost model was trained using a learning rate set at 0.001, an L1 regularization value of 5, an L2 regularization value of 2, 2,000 estimators or runs (model learning iterations), and a maximum depth of 1,000. Random forest, on the other hand, is a supervised learning algorithm employed for both classification and regression tasks (26). The random forest algorithm was trained with 2,000 decision trees (estimators), a depth of 1,000, and default values for other parameters. On the other hand, the multilayer perceptron represents a class of neural networks and serves as the fundamental form of a deep neural network, comprising a series of fully connected layers. These models are employed to address the typically high computing power demands associated with state-of-the-art deep learning architectures.
Data augmentation was conducted before initiating model training to mitigate class imbalance. Classifying imbalanced data may introduce bias in favor of the majority class, leading to suboptimal performance on the minority class. The Synthetic Minority Oversampling Technique (SMOTE) was employed on the dataset to address class imbalance. SMOTE is an oversampling method that involves generating “synthetic” examples for the minority class. This is achieved by creating synthetic examples along the line segments connecting any or all of the KNNs of each minority class sample (27). This approach aids in mitigating the overfitting issue associated with random oversampling. Following class balancing through SMOTE, the data was divided into a train/test split of 70/30, with 70% allocated for training and 30% for testing.
Performance evaluation metrics
The performance of the trained models was assessed and compared using accuracy, precision (also referred to as positive predictive value), sensitivity (also known as recall), F1 score (the harmonic mean of sensitivity and precision), and receiver operating characteristic (ROC)-area under the curve (AUC) plot. These evaluation metrics were calculated from the model’s confusion matrix, which summarizes classification outcomes using TP (true positive), TN (true negative), FP (false positive), and FN (false negative) values, as shown in Eqs. [1-6].
Results
Pregnancy risk factor analysis
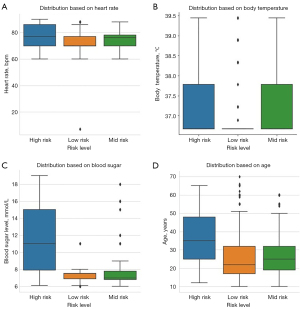
Figures 2,3 illustrate the distribution of pregnancy risk levels based on the risk factors [blood pressure (mmHg), heart rate (beats per minute), body temperature (℃), blood sugar level (mmol/L) and age] from the collected data. As indicated, high-risk factors are more visibly distributed at high systolic and diastolic blood pressures, high blood sugar levels and older ages.
The correlation between the pair of risk factors (features), and each risk factor with the high-risk level is demonstrated in Figure 4. Each cell in the correlation table shows the correlation between two variables (risk factors). As demonstrated, blood sugar level has the highest correlation with the high-risk level followed by diastolic and systolic blood pressure, while temperature has the lowest correlation with the high-risk level.

Results of risk level classification models
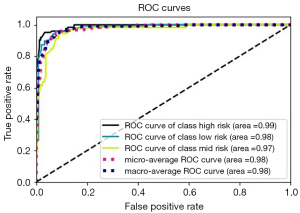
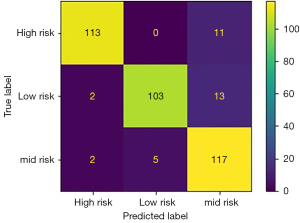
Summary of the trained classification models accuracy, precision, sensitivity, and F1 score is demonstrated in Table 2. The random forest classifier showed the highest performance in all evaluation metrics with 91% accuracy, 91.6% precision, 90.6% sensitivity and 91% F1 score in classifying the test data into high-risk, medium-risk and low-risk followed by KNN (88% accuracy). Hence, the random forest classifier was selected for implementation. The ROC curves of the random forest classifier evaluated on test data and the confusion matrix showing true positive and false negative values are demonstrated in Figures 5,6, respectively.
Table 2
| Models | Precision (%) | Sensitivity (%) | F1 score (%) | Accuracy (%) |
|---|---|---|---|---|
| KNN | 88 | 87.3 | 87.3 | 88 |
| MLR | 65 | 65 | 63.3 | 64 |
| SVM | 64 | 61.6 | 62 | 61 |
| MLP | 73 | 63 | 57 | 63 |
| XGBoost | 83 | 83 | 83 | 83 |
| Random forest | 91.6 | 90.6 | 91 | 91 |
KNN, K-nearest neighbor; MLR, multiclass logistic regression; SVM, support vector machine; MLP, multi-layer perceptron neural network; XGBoost, extreme gradient boosting.
User interface
For ease of use of the developed risk classification and pregnancy monitoring system, a user interface was developed using an open-source browser-based Python framework called ‘Streamlit’. The user interface has two main pages: pregnancy risk level prediction page (Figure 7) and pregnancy risk status monitoring (Figure 8). The risk level prediction system accepts patient’s age, systolic and diastolic blood pressure, blood sugar level, body temperature and heart rate, and presents the predicted risk level along with the percentage of prediction. Users can then record the clinical parameters to an existing patient or a new patient as a history file. Using the ‘pregnancy risk status monitoring page’, users can select a patient name from the drop-down list and observe the trend of each clinical parameter graphically from initial record date to the present date. This allows physicians or caregivers to track the trend of each parameter, monitor which parameters went beyond the normal range, throughout the pregnancy period and to monitor and make an informed decision.
Discussion
Every year, many pregnant women in underdeveloped countries, notably in Sub-Saharan Africa, die from pregnancy-related morbidities (28). The common leading causes of pregnancy-related mortality and morbidity are the pregnancy complications including blood pressure, gestational diabetes, preeclampsia (29), anemia, infections etc. It might sometimes be difficult to tell the difference between normal pregnancy symptoms and symptoms of problems. Early detection and proper management of these pregnancy-related risk problems is critical for providing effective care to the pregnant women. For effective results, maternal risk assessment and the subsequent management should be guided by evidence-based practice. However, with limited resources and experts, which are the cases of least developed countries, early pregnancy risk assessment and the resulting management is not up to the level of the burden and sensitivity of the issue.
The integration of digital technology into healthcare systems has opened the door for the potential enhancement of disease diagnosis and management through AI powered clinical decision support systems. These systems have the capability to improve accuracy, speed, and efficiency. One fundamental advantage of AI or machine learning systems is their objectivity, as they rely on real-world data and results, identifying crucial variables for physicians. AI stands poised to revolutionize women’s healthcare by enhancing diagnosis, alleviating physician workload, reducing healthcare costs, and offering benchmark analysis for tests with notable interpretation discrepancies among specialists. This holds particular significance for developing countries where expertise and resources are often limited.
This paper introduces a machine learning-driven system for classifying pregnancy risk levels and monitoring status using vital clinical parameters. The analysis and training of machine learning models for pregnancy risk level classification, as well as the development of the pregnancy risk status monitoring system, were conducted using data comprising age, systolic and diastolic blood pressure, blood sugar level, body temperature, and heart rate.
The analysis from our data indicates that high pregnancy risk is highly associated with higher systolic blood pressure, diastolic blood pressure, blood sugar level and older (Figures 2,3). This is evident that high blood pressure can cause a variety of health concerns, including cardiovascular disease, and it can disrupt the placenta’s development, limiting the baby’s nutrient and oxygen supply (30-33). The chance of having high blood pressure (hypertension) also increases with age and presence of diabetes mellitus (34-37). On the other hand, high blood glucose levels during pregnancy can potentially lead to premature labor, excessive fetal weight, or newborn respiratory issues after birth (38,39). This is also demonstrated in correlation plot of Figure 4, with blood sugar level having the highest correlation with the high-risk level followed by diastolic and systolic blood pressure, while temperature has the lowest correlation compared to other clinical parameters analyzed from the data used in this study. This indicates that the most important features for discriminating pregnancy risk levels, according to our data, are blood sugar level, systolic blood pressure and diastolic blood pressure.
The proposed machine learning based pregnancy risk level classification and monitoring system can be used as a decision support system to predict pregnancy risks early and manage them properly. Using the user interface, the physicians/obstetricians can feed vital clinical parameters to the classification system and automatically determine the risk level of pregnancy. The system will return the percentage of prediction probability for each class to allow the user to make an informed decision based on the results. The results along with the clinical records can also be saved for later reference and as an input to the pregnancy risk status monitoring system. Using the risk status monitoring system, user can regularly follow up the trends of each clinical parameter graphically throughout pregnancy period and monitor which parameters went below or above the normal ranges. This is significant to early detect health problems that could arise at any period of pregnancy, provide evidence-based treatment and increase the chance for normal pregnancy and a healthy baby’s birth. We acknowledge the need for a model trained on extensive and diverse datasets before it can be applied in clinical settings.
In conclusion, the proposed system can serve as a decision support tool, aiding healthcare professionals in the early prediction of pregnancy risk levels and improving pregnancy management. Moreover, it can be utilized to maximize the distribution of information to physicians/obstetricians at a point in the clinical workflow where decision-making is most effective. We acknowledge that further research by including a greater number of clinical parameters on a preferably much large number of data is required for optimal use of the proposed system.
Conclusions
This paper introduces a machine learning-based system for classifying pregnancy risk levels and monitoring status using clinical parameters. The study analyzed the correlation between clinical parameters and high pregnancy risk, identifying blood sugar level, systolic blood pressure, and diastolic blood pressure as crucial features for risk discrimination. Six machine learning models were trained and validated on a dataset of 1,014 entries, consisting of age, blood pressure, blood glucose level, body temperature, and heart rate for pregnancy risk prediction. The random forest model, which achieved a classification accuracy of 91%, was chosen for risk level classification. A user-friendly web-based interface was developed for both the pregnancy risk level classification and status monitoring systems, serving as a decision support tool for healthcare professionals in early pregnancy risk prediction and management.
Acknowledgments
Resources required to conduct the study were provided by the School of Biomedical Engineering and Faculty of Computing, Jimma Institute of Technology, Jimma University.
Footnote
Reporting Checklist: The authors have completed the TRIPOD reporting checklist. Available at https://ht.amegroups.com/article/view/10.21037/ht-23-10/rc
Data Sharing Statement: Available at https://ht.amegroups.com/article/view/10.21037/ht-23-10/dss
Peer Review File: Available at https://ht.amegroups.com/article/view/10.21037/ht-23-10/prf
Funding: None.
Conflicts of Interest: Both authors have completed the ICMJE uniform disclosure form (available at https://ht.amegroups.com/article/view/10.21037/ht-23-10/coif). The authors have no conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved. The study was conducted in accordance with the Declaration of Helsinki (as revised in 2013). This research did not involve humans, animals, or other subjects. According to Jimma University’s Institutional Review Board (IRB), no formal ethics approval was required in this particular case. Because of the retrospective nature of the research, the requirement for informed consent was waived.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
- World Health Organization. Trends in maternal mortality 2000 to 2020: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. World Health Organization; 2023.
- Nyamtema AS, Urassa DP, van Roosmalen J. Maternal health interventions in resource limited countries: a systematic review of packages, impacts and factors for change. BMC Pregnancy Childbirth 2011;11:30. [Crossref] [PubMed]
- World Health Organization. Trends in maternal mortality: 1990 to 2008: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. World Health Organization; 2010.
- Hogan MC, Foreman KJ, Naghavi M, et al. Maternal mortality for 181 countries, 1980-2008: a systematic analysis of progress towards Millennium Development Goal 5. Lancet 2010;375:1609-23. [Crossref] [PubMed]
- Say L, Chou D, Gemmill A, et al. Global causes of maternal death: a WHO systematic analysis. Lancet Glob Health 2014;2:e323-33. [Crossref] [PubMed]
- Musarandega R, Nyakura M, Machekano R, et al. Causes of maternal mortality in Sub-Saharan Africa: A systematic review of studies published from 2015 to 2020. J Glob Health 2021;11:04048. [Crossref] [PubMed]
- CDC. Pregnancy complications. 2019 [cited 2022 March 24]. Available online: https://www.cdc.gov/maternal-infant-health/pregnancy-complications/index.html
- Maranto M, Gullo G, Bruno A, et al. Factors Associated with Anti-SARS-CoV-2 Vaccine Acceptance among Pregnant Women: Data from Outpatient Women Experiencing High-Risk Pregnancy. Vaccines (Basel) 2023;11:454. [Crossref] [PubMed]
- Maranto M, Zaami S, Restivo V, et al. Symptomatic COVID-19 in Pregnancy: Hospital Cohort Data between May 2020 and April 2021, Risk Factors and Medicolegal Implications. Diagnostics (Basel) 2023;13:1009. [Crossref] [PubMed]
- Lennox CE. Assessment of obstetric high risk factors in a developing country. Trop Doct 1984;14:125-9. [Crossref] [PubMed]
- National Academies of Sciences, Engineering, and Medicine; Health and Medicine Division; Division of Behavioral and Social Sciences and Education, et al. Birth Settings in America: Outcomes, Quality, Access, and Choice. Washington (DC): National Academies Press (US); February 6, 2020.
- Fellows GF, Chance GW. High risk pregnancy: detection and management. Can Fam Physician 1982;28:1553-7. [PubMed]
- Rajbanshi S, Norhayati MN, Nik Hazlina NH. High-risk pregnancies and their association with severe maternal morbidity in Nepal: A prospective cohort study. PLoS One 2020;15:e0244072. [Crossref] [PubMed]
- Nyfløt L, Sitras V. Strategies to reduce global maternal mortality. Acta Obstet Gynecol Scand 2018;97:639-40. [Crossref] [PubMed]
- Goldie SJ, Sweet S, Carvalho N, et al. Alternative strategies to reduce maternal mortality in India: a cost-effectiveness analysis. PLoS Med 2010;7:e1000264. [Crossref] [PubMed]
- Prual A, Toure A, Huguet D, et al. The quality of risk factor screening during antenatal consultations in Niger. Health Policy Plan 2000;15:11-6. [Crossref] [PubMed]
- de Groot AN, Slort W, van Roosmalen J. Assessment of the risk approach to maternity care in a district hospital in rural Tanzania. Int J Gynaecol Obstet 1993;40:33-7. [Crossref] [PubMed]
- Jordan RG, Murphy PA. Risk assessment and risk distortion: finding the balance. J Midwifery Womens Health 2009;54:191-200. [Crossref] [PubMed]
- Kolluru V, Reddy A. Study of high risk scoring in pregnancy and perinatal outcome. Indian Journal of Obstetrics and Gynecology Research 2016;3:407-9.
- Goodwin JW, Dunne JT, Thomas BW. Antepartum identification of the fetus at risk. Can Med Assoc J 1969;101:57-passim. [PubMed]
- Burstyn I. Antepartum risk score predicts adverse birth outcomes. J Obstet Gynaecol Can 2010;32:16-20. [Crossref] [PubMed]
- Osheroff J, Teich J, Levick D, et al. Improving outcomes with clinical decision support: an implementer’s guide. 2nd Edition. Himss Publishing; 2012.
- Sutton RT, Pincock D, Baumgart DC, et al. An overview of clinical decision support systems: benefits, risks, and strategies for success. NPJ Digit Med 2020;3:17. [Crossref] [PubMed]
- Ahmed M, Kashem MA, Rahman M, et al. Review and Analysis of Risk Factor of Maternal Health in Remote Area Using the Internet of Things (IoT), In: InECCE2019. Lecture Notes in Electrical Engineering. Springer; 2020:357-65.
- Chen T, Guestrin C. Xgboost: A scalable tree boosting system. in Proceedings of the 22nd acm sigkdd international conference on knowledge discovery and data mining. 2016.
- Breiman L. Random Forests. Machine Learning 2001;45:5-32. [Crossref]
- Bishop CM, Nasrabadi NM. Pattern recognition and machine learning. New York: Springer; 2006.
- World Health Organization. Trends in maternal mortality 2000 to 2017: estimates by WHO, UNICEF, UNFPA, World Bank Group and UNDESA/Population Division. World Health Organization; 2019.
- Margioula-Siarkou G, Margioula-Siarkou C, Petousis S, et al. The role of endoglin and its soluble form in pathogenesis of preeclampsia. Mol Cell Biochem 2022;477:479-91. [Crossref] [PubMed]
- Arnadottir GA, Geirsson RT, Arngrimsson R, et al. Cardiovascular death in women who had hypertension in pregnancy: a case-control study. BJOG 2005;112:286-92. [Crossref] [PubMed]
- Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: systematic review and meta-analysis. BMJ 2007;335:974. [Crossref] [PubMed]
- Garovic VD, Bailey KR, Boerwinkle E, et al. Hypertension in pregnancy as a risk factor for cardiovascular disease later in life. J Hypertens 2010;28:826-33. [Crossref] [PubMed]
- Krielessi V, Papantoniou N, Papageorgiou I, et al. Placental Pathology and Blood Pressure's Level in Women with Hypertensive Disorders in Pregnancy. Obstet Gynecol Int 2012;2012:684083. [Crossref] [PubMed]
- Grundy SM, Cleeman JI, Daniels SR, et al. Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation 2005;112:2735-52. [Crossref] [PubMed]
- Yang Z, Xing X, Xiao J, et al. Prevalence of cardiovascular disease and risk factors in the Chinese population with impaired glucose regulation: the 2007-2008 China national diabetes and metabolic disorders study. Exp Clin Endocrinol Diabetes 2013;121:372-4. [Crossref] [PubMed]
- Hinkosa L, Tamene A, Gebeyehu N. Risk factors associated with hypertensive disorders in pregnancy in Nekemte referral hospital, from July 2015 to June 2017, Ethiopia: case-control study. BMC Pregnancy Childbirth 2020;20:16. [Crossref] [PubMed]
- Weissgerber TL, Mudd LM. Preeclampsia and diabetes. Curr Diab Rep 2015;15:9. [Crossref] [PubMed]
- Mimouni F, Miodovnik M, Siddiqi TA, et al. High spontaneous premature labor rate in insulin-dependent diabetic pregnant women: an association with poor glycemic control and urogenital infection. Obstet Gynecol 1988;72:175-80. [PubMed]
- Barnett AH, Stubbs SM, Mander AM. Management of premature labour in diabetic pregnancy. Diabetologia 1980;18:365-8. [Crossref] [PubMed]
Cite this article as: Simegn GL, Degu MZ. Maternal health risk analysis, automated pregnancy risk level identification and status monitoring system based on multivariable clinical data. Health Technol 2025;9:1.






